X. D. ZHOU
Department of Hydraulic Engineering and Water Resources, Xian University of
Technology, Xian, China
S. C. KOT
Research Center, Hong Kong University of Science and Technology, Hong Kong
ABSTRACT
This paper studies the adsorption characteristics of heavy metal ions onto suspended particles and bottom sediments of the Weiho and Hanjiang Rivers in China. These two rivers are chosen for their vastly different concentration of suspended matter. Adsorbents are suspended particles and bottom sediments of typical sections of these two rivers. Adsorbates are four heavy metal ions Cd, Zn, Cu, and Pb. Adsorption experiments were used to study effects of environmental factors, such as concentration of sediment, temperature, total ion number, pH value, different sediments, and different heavy metal ions, on the adsorption of heavy metal ions onto sediments. Differences and similarities of adsorption of heavy metal ions onto Weiho and Hanjiang River sediments are compared. Statistical analysis of the adsorption parameter A of heavy metal ions on suspended particles and bottom sediments was also carried out.
INTRODUCTION
There are close relationships between heavy metal ions and sediments, such as suspended particles, bed loads, and bottom sediments, in river water. River sediments adsorb most of the heavy metal ions in river water. In recent years, many researchers have investigated the adsorption of heavy metal ions onto river sediments or soils. Salim (1983) has studied the effects of the chemical composition and particle size of suspended in river water on the adsorption of lead onto these particles. Xiaoyuon (1983) has studied adsorption of heavy metal ions Cu, Zn, and Ni onto sediments in the Jionshajing river, and the results showed that the pH of river water is an important factor for adsorption heavy metal ions onto sediment. Walter (1974) studied the adsorption of hydrated compounds onto river sediment. The experimental results showed that carbon is the main factor to determine the adsorption value of heavy metal onto sediment. Stumm (1960), Smith (1972), Jinsheng (1987), Kwanshen (1984), and Xianchan (1987) have also studied the adsorption of heavy metal ions onto sediment.
This paper will discuss the experimental results of adsorption characteristics of heavy metal ions Cu, Cd, Pb, Zn onto Weiho and Hanjiang river sediments. We studied the effects of environmental factors, such as concentration, temperature, pH value, and total ion number on the adsorption of heavy metal ions onto sediment. The Weiho river is the main tributary of the Yellow River. Because of the industrial cities of Xian, Baoji, Sianyian, and Tianyuei located in the Weiho River valley, water pollution is very serious. Weiho is a typical river with a high concentration of sediment. In flood time the concentration of sediment is more than one hundred kilograms per cubic meter. The Weiho River tributary has North China valley characteristics. The concentration of sediment is high and the diameter of sediment is small. The Hanjiang is a main tributary of Chanjiang River. Its concentration of sediment is low under normal conditions. It is important to study the adsorption characteristics of heavy metal ions onto sediment to find out the special adsorption characteristics of rivers with high concentrations of sediment to set up water quality models for heavy metal and determine the heavy metal pollutant tolerance. So far nothing has been reported on the adsorption characteristics of Weiho and Hanjiang River sediments, especially on the adsorption of heavy metal ions onto high concentrations of sediment.
EXPERIMENTAL METHODS
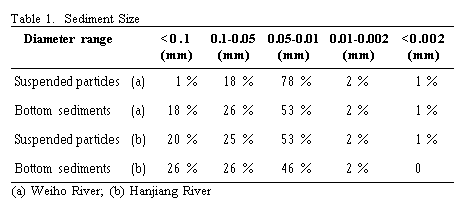
Sediment samples were obtained from the Baojixia hydrological station on the Weiho River and from the Yuohousi hydrological station on the Hanjiang River. Adsorbates were four metal ions Cu, Cd, Pb, and Zn. Adsorbents were suspended particles and bottom sediments. Sediment samples were prepared and dispersed in original river water and distilled water. The pH was adjusted in the range 1.5 to 13 with microliter additions of NaOH or HCl. Known volumes of heavy metal solution were added to produce final concentrations of heavy metals in the reaction flasks in the range of 10-80 ppm. The flasks were shaken at different temperatures for 3 hours and the solid phase separated by filtration through a 0.45 mm membrane filter. The quantity of metal adsorbed onto the solid was calculated by the difference between the amount of metal added and the final soluble concentration. The filtrates were analyzed for heavy metals concentration using the conventional method of anodic stripping voltammetry. Particle sizes have been determined using the sedimenting velocity method, and the results are shown in Table 1.

ADSORPTION CHARACTERISTICS OF WEIHO RIVER SEDIMENT
In north-west China, the rivers have high concentrations of suspended particles and bed loads. In the past, many adsorption experiments were done with low concentrations of sediment (S < 1.0 kg/m3) and the results have some limitations. For this adsorption study the range of concentration of sediment was from 1 g/l to 35 g/l.
Adsorption Capacity of Cu on Sediment
In general, the capacity of suspended particles to adsorb pollutants increases with concentration of sediment. The adsorptive capacity of sediment, Cw, is the adsorption value of heavy metal ions on suspended particles for a unit water volume. Figure 1 shows the results of adsorptive capacity of Cu onto suspended particles where the ordinate is the adsorptive capacity, Cw, and the abscissa is the concentration, G, of Cu under equilibrium conditions. We can see from Figure 1 that the adsorptive capacity of suspended particles increases with the increase of sediment concentration for the same Cu concentration at equilibrium. Adsorptive capacity also increases with the increase of G with the same concentration of sediment. The rate of increase Cw is slowed as G increases. Finally the rate of increase of Cw levels off and the adsorptive capacity approaches a constant value. We can describe the relationship of Cw and G using Langmuir's isotherm,

where Bm is the saturation adsorptive capacity, Am is the adsorption parameter correlated with adsorptive capacity. For different concentrations of suspended particles, the saturation adsorptive capacity, Bm, and adsorption parameter, Am, are different. According to the analysis of experimental results, we can find variation parameters Bm and Am shown in Figure 2 and Figure 3 as a function of concentration. Saturation adsorptive capacity is a linear relationship to concentration of suspended particles, and adsorptive parameter has a U type relationship to the concentration of suspended particles with a minimum at 20 g/l sediment concentration.
Adsorption Value of Unit Quantity Sediment
The adsorption value of unit weight of sediment is conceptually different from the adsorptive capacity of sediment. The main difference is that it exhibits the effect of concentration of sediment on adsorption. Figure 4 is the adsorption value, Cs, of Cu on unit weight of suspended particles. From this Figure, we can see that adsorption value decreases when sediment concentration increases. We can also describe the relationship between Cs and G using Langmuir's isotherm, that is:

where B is saturation adsorption value and A is the adsorption factor. According to the analysis of experimental results, we can find B and A as a function of concentration of sediment as shown in Figures 5 and 6. From Figure 5, we can see that the saturation adsorption value behaves differently from saturation adsorptive capacity. B decreases when concentration of sediment, S, increases. When S is less than 6 g/l, B decreases when S increases; when S is more than 6 g/l, B is constant. In Figure 6 we can see that A behaves similarly as Am.
Temperature Effect On Adsorption Value
We have studied the effect of temperature on adsorption value of Cu onto suspended particles and the experimental results are shown in Figure 7. We can see that the adsorption value increases with temperature in the same equilibrium Cu concentration. The reason is that as temperature increases, the adsorption activation energy of Cu onto sediment increases, and also the adsorption entropy. The adsorptive ability increases with adsorption entropy.
Effect of Sediment Type on Adsorption Value
We have studied the adsorption value of Cu onto Weiho River suspended particles and bottom sediment. Experimental results are shown in Figure 8. We can see that adsorption value of Cu onto suspended particles is three times the value for bottom sediment. As the samples of suspended particles and bottom sediment are from the same river section, their chemical compositions are approximately the same, but their particles sizes are different. So the main reason for the differences in their adsorption values is their size. In real river flow, the bottom turbulent stress is large enough to suspend the small diameter sediments and carry them downstream. The large diameter sediment remains in place. In general, the size of suspended particles is smaller than that of bottom sediment. For the Weiho River, the medium diameter of suspended particles (d50)is 0.016 mm, and the medium diameter of bottom sediment (d50)is approximately 0.0266 mm. The smaller the sediment diameter, the more surface area per gram sediment is exposed, and therefore the adsorptive ability is large. The adsorption value of Cu onto suspended particles is understandably larger than that onto bottom sediment.
Effect of Different Heavy Metal Ions on Adsorption Value
According to experimental results we have mapped the adsorption isotherms of suspended particles adsorbing Cu, Cd, Pb and Zn. These are shown in Figure 9. The adsorption value of Zn is the largest, then in descending order are Pb, Cd, and Cu. The adsorption values of Cd and Cu show only a small difference. When the heavy metal ions equilibrium concentration is lower than 35 ppm, the adsorption value of Cu on suspended sediment is a little smaller than that of Cd. In general, the adsorption value of Pb is 1.5 times that of Cu and Cd onto suspended particles, and the adsorption value of Zn is 3 times of Cd and Cu. Figure 10 shows the experimental results of the adsorption of Zn, Pb, Cd, and Cu onto bottom sediment. From Figure 10 we can see the decreasing order of adsorption value of the four heavy metal ions on bottom sediment for large G, but the difference in values is not large. For saturation adsorption value, the adsorption value of Cd is 1.1 times of that of Cu, and the Pb value is 2 times of that of Cu on bottom sediment. The adsorption value of Zn is more than 1.5 times Pb on bottom sediment. Comparing the experimental result of Figure 8 and Figure 9, the adsorption values of the four heavy metal ions on bottom sediment are found to be less than the values for suspended particles, as discussed before.
Effect of Total Ion Number on Adsorption Value of Pb onto Suspended Particles
Figure 11 shows that total ion number affects the adsorption value of Pb onto suspended particles. It shows that the adsorption value of Pb onto suspended particles in distilled water (ion numbers are low) is more than that of Pb onto suspended particles in original Weiho river water. There are two reasons. First, the higher total ion number is due the higher concentration of Ca, Mg, K and Na, which compete with Pb. Second, in high total ion number water, complex compounds of Pb combining with Cl, and SO4 can be formed more easily.
ADSORPTION CHARACTERISTICS OF HANJIANG RIVER SEDIMENT
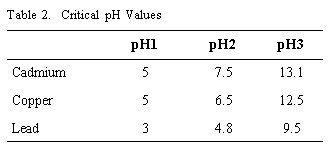
The pH value is an important parameter which affects adsorption of heavy metal on sediment. Our adsorption experiments were performed in water of pH values from 1 to 12 with microliter additions of NaOH or HCl. Adsorbates are Pb, Cd, and Cu. The influence of the pH value on the adsorption of Pb ions onto suspended particles is shown in Figure 12. The concentration of dissolved Pb ions decreases with the increase of pH value. For a solution without sediment, when the pH value increases, it causes hydrolysis and sedimenting of lead ions. The concentration of dissolved lead ions decreases, approaching zero when the pH value is more than 9.5. For a solution with sediment, because of sediment adsorption, when the pH value is small, the concentration of dissolved lead ions will also decrease. This result is similar to Xiaoyuong's (1983) experimental results using soil adsorbing Cd. From Figure 12 we can see that when pH is less than 3.0, the adsorption value, Cs, is constant, approximately equal 1.0 mg/g; when pH is more than 4.8, the Cs value increases with pH increase; when pH is 4.8, Cs is a maximum value, approximately equal 2.15 mg/g. When pH is more than 4.8, Cs decreases with pH increase, and when pH is more than 9.5, Cs approaches zero. Figure 13 shows the experimental result of the influence of pH on adsorption of Cd onto suspended particles. When pH is less than 5, Cs is constant, approximately equal to 1.5 mg/g; when 5 < pH < 7.5, Cs increases, and Cs approaches zero when pH is more than 13. Figure 14 shows the experimental results of influence of pH on the adsorption of cadmium ions onto suspended particles which is similar to lead ions and cadmium ions. A critical value of pH is 6.5, where the maximum value of adsorption of copper ions onto suspended particles is 1.65. When pH is less than 5, the adsorption value is constant. From these experimental results we can conclude that the influence of pH on adsorption of heavy metal ions onto sediment is large. There are three stages of adsorption values as pH increases from 2 to 14. When pH < pH1, Cs is equal to constant; when pH = pH2, Cs is equal to a maximum value; when pH > pH3, Cs is equal to zero. When pH is less than pH2, the main activity is sediment adsorbing heavy metal ions, so Cs may increase with pH. When pH > pH2, the main activity is hydrolysis and sedimenting of heavy metal ions. The critical pH value (pH2)is a function of concentration of heavy metals ions and hydrolysis condition. In our experimental conditions, pH1, pH2, pH3 values are shown in Table 2.
Influence of Different Coexisting Heavy Metal Ions on Adsorption
Figure 15 shows the experimental results of influence of different coexisting heavy metal ions on adsorption of heavy metal ions onto suspended sediment. The adsorption value of Cu onto suspended particles in Cd + Cu solution is less than that of Cu onto suspended particles in a solution with Cu alone. The reason is that the sediment area is constant while the heavy metal ions number is doubled when two heavy metal ions are present. The adsorption value of heavy metal ions onto sediment does not automatically double. The increase in rate of adsorption value is less than the increase in rate of heavy metal ions number. The adsorption value in the presence of other heavy metal ions of different species is less than that in solution of only one single species of heavy metal ion. Figure 16 shows the influence of adsorption value of Cd onto suspended particles with Cd + Cu coexisting and Cd + Zn coexisting. From this figure, we can see that the adsorption value of Cd when only Cd exists is more than that of Cd onto sediment with Cd + Cu coexisting and Cd + Zn coexisting. The adsorption value of Cd onto sediments with Cd + Zn coexisting is higher than the adsorption value of Cd onto sediment with Cd + Cu coexisting. This is because the adsorption value of Zn is more than the adsorption value of Cu, so part of surface area of sediment is occupied by Zn with Zn + Cd coexisting, and only a smaller area is occupied by Cd. The area of sediment occupied by Cu with Cu + Cd coexisting is less than the area of sediment occupied by Cd. Then the adsorption value of Cd onto sediment with Cu + Cd coexisting is larger.
Influence of Sediment Type on Adsorption
The experimental results of adsorption of Pb onto sediment and suspended particles shown that for Zn and Pb, the adsorption value onto suspended particles is higher than that onto sediment (Figures 17 and Figure 18). The reason is that the average radius of suspended particles is less than that of sediment.
COMPARISON OF HEAVY METAL ADSORPTION CHARACTERISTICS ONTO WEIHO AND HANJIANG RIVER SEDIMENTS
The Weiho and Hanjiang Rivers are two different types of river. Weiho is a river with a high concentration of sediment while Hanjiang is a river with a low concentration of sediment. The Weiho and Hanjiang valleys have different geological, geochemical, and climatic conditions. The mineral composition, chemical characteristics, size distribution, and inorganic matter of sediments are very different. All these affect the adsorption of heavy metal onto sediment.
Comparison of Adsorption Character of Zn
The experimental results of the adsorption value of Zn onto Hanjiang and Weiho River suspended particles show that the adsorption value of Zn onto suspended particles in the Weiho river is higher than that of Zn onto suspended sediment in the Hanjiang River (Figure 19). The Cs for Weiho bottom sediment is slightly higher that of the Hanjiang bottom sediment (Figure 20).
Comparison of Adsorption Value of Pb onto Sediment
Figure 21 shows the adsorption value of Pb onto suspended particles in the Weiho River is higher than that of the Hanjiang River.
STATISTICAL ANALYSIS OF SEDIMENT ADSORPTION PARAMETER, A
From the experimental results of the Weiho and Hanjiang rivers sediment adsorption of heavy metal ions, we can see that they approximately follow Langmuir's isotherm. In equation (1) and (2), let Cw or W = Cs/B, then we have:

where W is the relative adsorption value. The correlation of W and G is given in Figure 22 which shows that the adsorption of heavy metal ions onto sediment for different sediment types, different heavy metals, and different environmental factors, almost all of which follow equation (3). Relative adsorption value, W, increases with G, and approaches 1.0 whenG is very large. Their difference is indicated by the parameter A.
The computation of A according to these experimental results shows that A varies from 0.04 to 3.0. In Figure 23 the ordinate is the probability density function of A (F(A)), and the abscissa is the value of A. From this figure, we can see that the distribution of the adsorption factor A is concentrated, the main distribution range being from 0.1 to 1.0 ppm. the critical value of A is 0.25 ppm. When A equals 0.25 ppm, F(A) is at the maximum value, approximately equal to 1.9. In the range 0.04 ppm < A < 0.025 ppm, F(A) sharply increases with A, from 0.0 to 1.9. From 0.25 ppm < A < 1.0 ppm, F(A) sharply decreases when A increases, from 1.9 to 0.36 ppm. For A > 1.0 ppm, F(A) gradually decreases when A increases, approaching zero when A > 2.5 ppm.
CONCLUSION
This paper has studied the adsorption characteristics of the heavy metal ions Cd, Cu, Zn, Pb onto Weiho and Hanjiang River bottom sediments and suspended particles. The conclusions reached are:
REFERENCES
Salim, R.; Adsorption of lead on the suspended particles of river water, Water Research, Vol. 17, No. 4, 1983.
Xiaoyuong, Wang; Adsorption of heavy metals on Jinshajiang river sediment, Environmental Chemistry, Vol. 2, No. 1, 1983.
Walter, L.J.; Mercury occurrence on sediment cores from western lake Erie, Ohio, J. Sci. 74
Stumm, W.; The chemistry of aqueous iron, Schweiz. Z. Hydrol. 21, 1960.
Smith, P.A.; The distribution of trace metal in the surficial sediment surrounding Keweenaw Point, Upper Michigan, Proc. 15th Conf. Great Lake Res., 1972.
Jingsheng, Chen; A study on heavy metal partitioning in sediments from Poyang Lake in China, Hydrobiologia 176/177: 159-170, 1987.
Kwanshen, Gao; Adsorption of Cadmium ions on China main river suspended particles, Environmental Chemistry, Vol. 2, No. 4, 1984.
Xianchan, Zin; Adsorption and desorption rate of Hg and Cd on Xianjiang River sediment, China Environmental Science, Vol. 7, No. 2, 1987.
X.D. ZHOU
Department of Hydraulic Engineering and Water Resources
Xian University of Technology
Xian, China